Abstract
Introduction: Despite recent advances in treatment, patients with multiple myeloma (MM) continue to relapse. G protein-coupled receptor family C group 5 member D (GPRC5D) is a promising target for immunotherapy in patients with MM due to its high expression in malignant plasma cells and limited expression in normal human tissue; unlike other antigens targeted by MM therapies, there is no indication that GPRC5D sheds into the periphery. Talquetamab (JNJ-64407564) is a first-in-class bispecific IgG4 antibody that redirects T cells to kill MM cells by binding to both GPRC5D and CD3 receptors. Here we report updated and new results of talquetamab at the recommended phase 2 doses (RP2Ds) from a phase 1 trial in relapsed/refractory MM (RRMM; NCT03399799).
Methods: Eligible patients with MM had relapsed or refractory disease or were intolerant to standard therapies; patients previously treated with B-cell maturation antigen (BCMA)-directed therapies were eligible. This analysis focuses on patients who received talquetamab subcutaneously (SC; range 5.0-800 µg/kg) weekly or biweekly. Step-up dosing was used as a patient management strategy to minimize the severity of cytokine release syndrome (CRS). The primary objectives were to identify the RP2D (part 1) and assess talquetamab safety and tolerability at the RP2Ds (part 2). Adverse events (AEs) were graded by CTCAE v4.03 with CRS events graded per Lee et al 2014 criteria. Responses were investigator-assessed per International Myeloma Working Group criteria.
Results: As of July 19, 2021, 95 patients have received SC talquetamab. The RP2D was originally identified as a weekly SC dose of 405 µg/kg talquetamab with step-up doses. However, alternative dosing schedules that require less frequent administration continue to be investigated. A biweekly RP2D was also identified as an SC dose of 800 µg/kg talquetamab with step-up doses.
30 patients received the 405 µg/kg weekly dosing schedule (median age: 61.5 years [range 46-80]; 63% male; 100% triple-class exposed; 80% penta-drug exposed; 77% triple-class refractory, 20% penta-drug refractory; 30% prior BCMA-directed therapy; median follow-up: 7.5 mo [range 0.9-15.2]). 23 patients received the 800 µg/kg biweekly dosing schedule (median age: 60.0 years [range 47-84]; 48% male; 96% triple-class exposed; 70% penta-drug exposed; 65% triple-class refractory, 22% penta-drug refractory; 17% prior BCMA-directed therapy; median follow-up 3.7 mo [range 0.0-12.0]).
There were no treatment discontinuations due to AEs at either of the RP2Ds. The most common AEs at the 405 µg/kg weekly dose were CRS (73%; 1 patient had grade 3 CRS), neutropenia (67%; grade 3/4: 60%), and dysgeusia (60%; grade 2: 29%); skin-related AEs occurred in 77% (all grade 1/2; nail disorders: 30%) of patients, and infections occurred in 37% of patients (1 patient had grade 3 COVID-19 pneumonia). The most common AEs at the 800 µg/kg biweekly dose were CRS (78%; all grade 1/2), dry mouth (44%; all grade 1/2), and neutropenia (44%; grade 3/4: 35%); skin-related AEs occurred in 65% of patients (grade 3: 13%; nail disorders: 17%) and infections occurred in 13% of patients (1 patient had grade 3 pneumococcal sepsis).
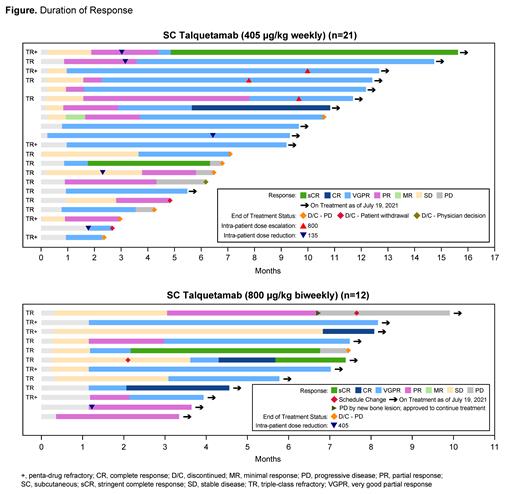
In 30 response-evaluable patients treated with the 405 µg/kg weekly dose, the overall response rate (ORR) was 70% (very good partial response or better [≥VGPR] rate: 57%). In 17 response-evaluable patients treated with the 800 µg/kg biweekly dose, the ORR was 71% (≥VGPR rate: 53%). Responses were durable and deepened over time in both cohorts (Figure). Median duration of response (DOR) was not reached at either RP2D; the 6-month DOR rate for patients who received the 405 µg/kg weekly dose was 67% [95% CI: 41-84]. Serum trough levels of talquetamab were comparable at both RP2Ds. Consistent with the mechanism of action for talquetamab, pharmacodynamic data from cohorts treated at both dose levels showed peripheral T-cell activation and induction of cytokines.
Conclusions: These findings indicate that SC talquetamab is well tolerated and highly effective at both RP2Ds. Preliminary data from the 800 µg/kg biweekly cohorts indicate that less frequent, higher doses of SC talquetamab do not have a negative impact on the previously described safety profile. Further investigation of talquetamab as monotherapy (phase 2; NCT04634552) and in combination with other therapies in patients with RRMM is underway.
Krishnan: MAGENTA: Consultancy; BMS: Consultancy, Current equity holder in publicly-traded company, Speakers Bureau; JANSSEN: Consultancy, Research Funding; City of Hope Cancer Center: Current Employment; REGENERON: Consultancy; SANOFI: Consultancy; GSK: Consultancy; Amgen: Speakers Bureau. Minnema: Celgene: Other: Travel expenses; Alnylam: Consultancy; Cilag: Consultancy; BMS: Consultancy; Janssen: Consultancy; Kite/Gilead: Consultancy. Berdeja: Lilly, Novartis: Research Funding; Abbvie, Acetylon, Amgen: Research Funding; Celularity, CRISPR Therapeutics: Research Funding; EMD Sorono, Genentech: Research Funding; Poseida, Sanofi, Teva: Research Funding; Bluebird bio, BMS, Celgene, CRISPR Therapeutics, Janssen, Kite Pharma, Legend Biotech, SecuraBio, Takeda: Consultancy; GSK, Ichnos Sciences, Incyte: Research Funding. Oriol: Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. van de Donk: Roche: Consultancy; Takeda: Consultancy; Cellectis: Research Funding; Amgen: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; BMS/Celgene: Consultancy, Honoraria; Novartis /bayer/servier: Consultancy. Rodriguez-Otero: Clínica Universidad de Navarra: Current Employment; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene-BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Regeneron: Honoraria. Askari: Janssen: Research Funding. Mateos: Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sea-Gen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene - Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria; Bluebird bio: Honoraria; AbbVie: Honoraria; GSK: Honoraria; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees. Costa: BMS: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Karyopharm: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Speakers Bureau. Verona: Janssen: Current Employment. Ma: Janssen: Current Employment, Current holder of individual stocks in a privately-held company. Girgis: Janssen: Current Employment, Current holder of individual stocks in a privately-held company. Yang: Janssen: Current Employment. Hilder: Janssen: Current Employment, Current holder of individual stocks in a privately-held company. Russell: Janssen: Ended employment in the past 24 months. Goldberg: Janssen: Current Employment, Current holder of individual stocks in a privately-held company. Chari: Shattuck Labs: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Millenium/Takeda: Consultancy, Research Funding; Sanofi Genzyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Antengene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Secura Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal